Proton Exchange Membranes
Proton Exchange Membranes
Hydrogen technologies
Hydrogen technologies relate to the production and use of hydrogen as part of the hydrogen economy¹. In this context, the hydrogen economy encompasses the production of hydrogen and the use of hydrogen in ways that contribute to phasing out fossil fuels and limiting climate change².

The most common methods for producing hydrogen on an industrial scale are steam reforming, oil reforming, coal gasification, and water electrolysis³. To produce hydrogen with a low environmental impact (green hydrogen), all pollutants must be processed or sequestered.
The four major primary energy sources to generate hydrogen (and the respective H2 production method) are⁴:
- Thermal: Thermal decomposition of water at high temperatures (thermolysis)
- Electrical: Direct current is used to split water into O2 and H2 (electrolysis)
- Photonic: PV panels are used to generate direct current, or water is split by using the electron-hole pairs (electrolysis or photocatalysis)
- Biochemical energy: Biological systems are used to generate H2 in the absence of light (dark fermentation).
Electrolysis of water is considered the most capable route to generate clean hydrogen because the process does not yield carbon emissions and only produces oxygen as a by-product³.
Proton Exchange Membrane Fuel Cell (PEMFC) and
Proton Exchange Membrane Water Electrolyser (PEMWE)
PEMFC has the potential to be an important player in hydrogen energy technologies. PEMFC is featured by low operational temperature, zero noise, fast start-up, high energy density, high electrical efficiency, and long operational lifespan³. PEMWE stands out due to its high production rate of pure hydrogen and high energy efficiency.


Figure 1. The main components and working principles of PEMFC and PEMWE are taken from Reference nr 3 under a Creative Commons license. The figure has been rearranged.
PEMFC produces electricity from the chemical reaction between hydrogen and the air and generates water as a by-product. It can be Low-Temperature (LT-PEMFC) operating at 60-80 °C or High-Temperature (HT-PEMFC) operating at 120 – 200 °C.
PEMWE is an electrochemical device that produces hydrogen from water using electrical energy and generates oxygen as a by-product.
The main components and materials of Proton Exchange Membrane (PEM) technologies such as PEMFC and PEMWE are⁵:
- Ion exchange membrane as a solid electrolyte. The ionic conductivity is the most important feature of the slot-die-coated solid polymer membrane. It should be durable, robust, and resistant to chemical attack at a wide range of operating temperatures.
- Catalyst layer. Its function is to initiate the electrochemical reaction. It can be slot-die coated on the ion exchange membrane. When the ion exchange membrane and the catalyst layer are commercially fabricated, they are known as membrane electrode assemblies (MEA).
- Electrically conductive, porous gas diffusion layer. This is the outer layer of the MEA, placed between the flow plates and the catalyst layer. Among other functions, it provides mechanical support, protects the catalyst layer from corrosion, contributes to heat removal, and helps disperse the reactant.
- Cell interconnects and flow/cooling plates. These deliver H2 or water to reactive sites via flow channels and electrically connect the cells. They provide mechanical support for the cells in a PEMFC or PEMWE stack.
The MEA is the heart of the PEM devices and can consist of three layers. It is a membrane with a catalyst layer coated on both sides, and then it is called a Catalyst-Coated membrane (CCM).

Figure 2. Low-temperature electrolysis testing stations in the hydrogen research area at the National Renewable Energy Laboratory’s (NREL’s) Energy Systems Integration Facility (ESIF)
The CCM can be produced, for example, by fabricating the membrane by roll-to-roll slot-die coating onto a peelable carrier substrate (PTFE is commonly used) and then slot-die coat the catalyst on each side of the membrane either sequentially using a FOM sigmaR2R or simultaneously using a FOM moduloR2R.

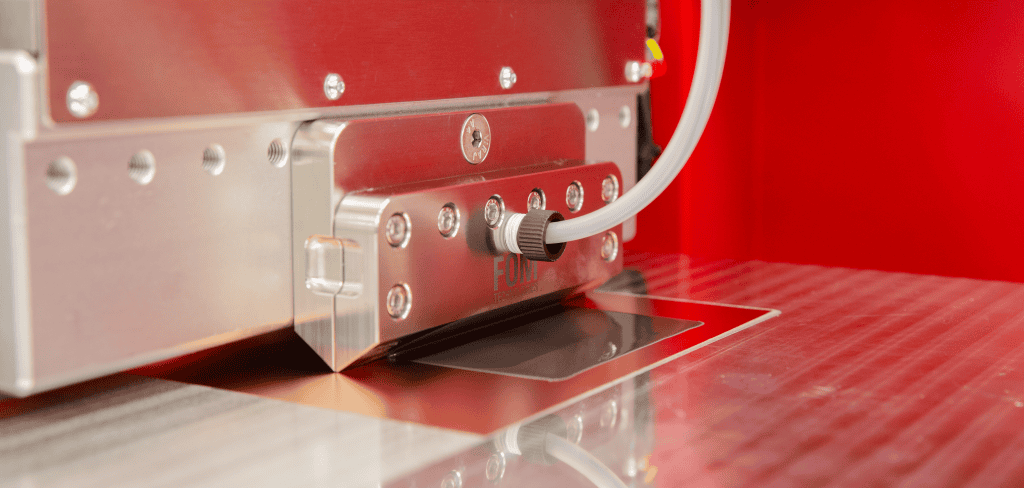
Figure 3. Slot-die coating of PVP:PSU blends to fabricate PEMs on a AlphaSC using a heated high viscosity syringe, heated high viscosity tubing+fittings and on a heated microporous hot plate.
One of the advantages of using a FOM moduloR2R is the possibility to design it case by case, adding modules for specialized additive manufacturing deposition techniques, paving the way for potentially solving the fabrication challenges of PEM devices.
FOM industrial research activities in Proton Exchange Membranes
FOM coordinates a MissionBooster industrial research project called the In-line characterization system to improve the quality and reproducibility of slot-die-coated polymer electrolyte membranes.
Granted by Innovation Fund Denmark (Grant no.: 2122-00062B)
In this project, FOM teams up with the Department of Energy Conversion and Storage from the Danish Technical University (DTU Energy) and the Danish National Metrological Institute (DFM) to develop a real-time roll-to-roll compatible characterization tool with specialized software and hardware for the quantitative assessment of thickness homogeneity and the presence of defects in multilayer PEM structures.
It will add specialized metrological capabilities to FOM’s R2R additive manufacturing modular production lines.




Figure 4. Roll-to-roll slot-die coating of PVP:PSU to fabricate PEM on a compact FOM R2R machine in the framework of FOM’s MissionBooster project with DFM and DTU.
References
- J. Yap and B. McLellan, “Exploring transitions to a hydrogen economy: Quantitative insights from an expert survey,” Int. J. Hydrogen Energy, vol. 66, pp. 371–386, 2024
- Hydrogen economy, Wikipedia
- A. Baroutaji, A. Arjunan, J. Robinson, M. A. Abdelkareem, and A.-G. Olabi, “Additive manufacturing for Proton Exchange Membrane (PEM) hydrogen technologies: merits, challenges, and prospects,” Int. J. Hydrogen Energy, vol. 52, pp. 561–584, Jan. 2024
- I. Dincer and C. Acar, “Review and evaluation of hydrogen production methods for better sustainability,” Int. J. Hydrogen Energy, vol. 40, no. 34, pp. 11094–11111, 2015
- A. Baroutaji, J. G. Carton, M. Sajjia, and A. G. B. T.-R. M. in M. S. and M. E. Olabi, “Materials in PEM Fuel Cells,” Elsevier, 2016
The image featured at the top banner is an illustration by Alfred Hicks/NREL, and it shows the Hydrogen Crossover Analysis in PEM Electrolysis Cells.
The graphic image of a green hydrogen power plant is from Shutterstock, and was created by a contributor Allahfoto.
Read more on the topic
Download the FOM Technologies Product brochure