Slot-die coating and Power-to-X
Slot-die coating and Power-to-X
A boom in Power-to-X investments
At FOM Technologies, we see the Green Transition accelerating around us every day. Investments and innovation are happening fast in well-established green energy fields such as solar energy harvesting and battery energy storage, where slot-die coating already plays a substantial role in conventional manufacturing, or holds promise for scaling the production of next-gen devices. Innovation in established fields is certainly exciting, but less established technologies are also being accelerated towards maturity through booming investments in green concepts and pilot projects.
In Denmark and internationally, Power-to-X (PtX) technologies are among the most prominent recent examples of this trend. With a projected market value of EUR 10 tn by 2050 for green hydrogen alone, as well as the potential to decarbonize existing industry and provide seasonal storage for renewable electricity, it’s no surprise that PtX processes are getting a moment in the spotlight. In this post, we’ll provide a brief overview of the PtX concept, as well as a discussion of how slot-die coating can play a critical role in bringing PtX processes to scale.

Avedøre Power Station near Copenhagen is proposed as an onshoring point for off-shore wind energy to be used in subsequent Power-to-X processes. (Image credit: Ørsted A/S)
What is PtX?
Before getting into the role of slot-die coating in the PtX puzzle, it first might help to describe what is meant by the term “Power-to-X.” Power-to-X is a collective term for technologies in which excess electricity (e.g., from variable renewable sources such as wind and solar) is transformed into hydrogen, synthetic gases, fuels or fine chemicals via a series of thermo-, photo-, or electrochemical conversion processes.

Power-to-X processes enable us to convert excess renewable electricity into variety of diverse chemical fuels and products. This is achieved via a combination of new electrolysis technologies and well-established industrial processes originally develop for fossil fuel-based inputs.
By using this excess electricity to produce fine chemicals, value is derived from energy that would otherwise be wasted or offloaded at an undesirable price. Alternatively, the electricity can be converted into chemical fuels rather than fine chemical products. In this way, the excess renewable energy can be cheaply stored as physical fuel for long periods of time more practically than via a battery-based alternative, while still resulting in zero carbon emissions.
The most attractive PtX output products
Technologies that convert excess electricity into other products therefore exist at the heart of the PtX concept. These conversion processes are sometimes categorized as “primary” and “secondary” conversion processes, depending on how many conversion steps are required to turn the excess electricity input into the desired output product.

Hydrogen is a key output from the (primary) PtX process of water electrolysis. This hydrogen product can then be applied in a variety of well-established (secondary) industrial processes for the production of more specialized fuels.
- Hydrogen: usable as an industrial reagent, fuel, or intermediate vector between primary and secondary PtX conversion processes (more on that later). Hydrogen is a widely used industrial reagent (in e.g. steel, fertilizer, and food processing) that is currently almost entirely produced from fossil fuels via steam methane reforming (SMR) and water-gas shift upgrading of the resulting syngas. Replacing conventional “grey” hydrogen production with “green” PtX production therefore reduces our dependence on fossil fuels and reduces the carbon footprint of existing industries. Hydrogen can itself be used as a green fuel source or upgraded into several other green fuels for specialized applications. For these reasons, hydrogen and its production are arguably the most important, central pieces of the overall PtX puzzle.
- Methane: usable as a fuel that can be easily integrated into existing natural gas infrastructure, allowing excess electricity to be stored as natural gas and used in conventional natural gas-driven processes and machinery. Methane is the primary component of natural gas, which is a conventional fossil fuel. PtX-based methane production therefore reduces our dependence on conventional fossil fuels.
- Synthetic natural gas and liquid hydrocarbon fuels: usable as a fuel in applications where high energy density hydrocarbons are required, for example in shipping and aviation, where batteries, hydrogen, and methane do not provide the required energy density for long-haul transport in a reasonable weight/footprint. Like methane, natural gas and liquid hydrocarbons are conventional fossil fuels. Producing them via clean PtX processes therefore also reduces our dependence on conventional fossil fuels.
- Methanol usable as a chemical reagent (for e.g., formaldehyde, formic acid, synthetic diesel, etc.) or liquid fuel. Methanol is the simplest carbon-based liquid fuel that can be produced from PtX processes. The fact that it is carbon-based makes it relatively simple to integrate into conventional infrastructure. Its liquid state under standard conditions gives it a reasonable volumetric energy density while also making it easy to store and transport than gaseous fuels. Almost all methanol is currently derived from fossil fuels via syngas from SMR (the same process that produces conventional hydrogen). Producing methanol via clean PtX processes therefore also reduces our dependence on conventional fossil fuels.
- Ammonia: usable as a chemical reagent, fertilizer, nitrogen-based liquid fuel, or hydrogen storage medium. It can be liquified relatively easily, offering an attractive energy density and hydrogen content in its liquid form. It offers many similar benefits to methanol as a fuel while also being carbon-free, representing a financial advantage over carbon-based fuels where upstream and downstream carbon capture processes are required. Which of these fuels achieves mainstream adoption will likely depend on financial considerations that are not yet set in stone. Ammonia has a well-established global production and supply chain infrastructure due to its widespread use as an agricultural fertilizer. Conventional ammonia production occurs via the Haber-Bosch process, which requires a significant amount of hydrogen and energy input. Because hydrogen is conventionally produced from fossil fuels, conventional ammonia production is a highly carbon-emitting process. However, this also means that replacing conventional hydrogen in the Haber-Bosch process with green PtX hydrogen enables manufacturing of much greener ammonia, sometimes termed “e-ammonia.” Producing ammonia via clean PtX processes therefore also reduces our dependence on conventional fossil fuels.
In summary, the most significant PtX products currently being targeted for scale-up are a variety of relatively simple molecules with clear applications as widespread industrial reagents or specialized fuels. As industrial reagents, these PtX products allow us to decarbonize our industries without requiring the invention of entirely new industrial processes. As fuels, they promise low-cost, long-term energy storage that takes advantage of existing infrastructure and meets needs that cannot be served by alternative storage technologies like batteries. Critically, hydrogen plays an important role as both a fuel and a reagent that can be upgraded into more specialized fuels through combination with other abundant molecules via well-established industrial processes.
Primary and secondary conversion processes
But how does slot-die coating fit into this complex narrative? To answer this question, it’s important to consider the relative utility of these output products, as well as the number and maturity of steps involved in producing them from excess electricity via PtX processes.
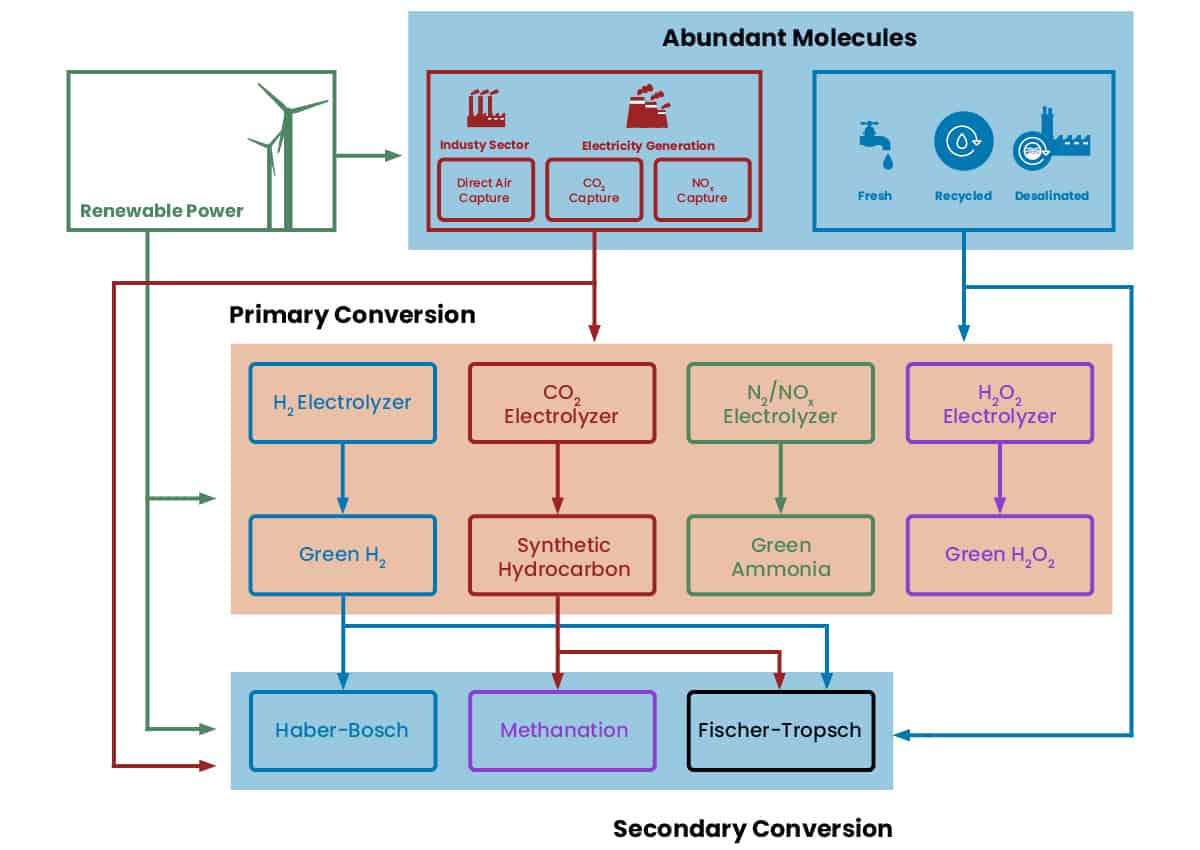
A wide variety of primary conversion processes (typically based on electrolysis) have been demonstrated at the R&D phase, though not all primary processes are equally mature or versatile. Pairing the most mature and versatile primary conversion processes with well-established secondary conversion processes currently holds the most promise for scale-up and industrialization.
This is where we can loop back to the concepts of primary and secondary conversion processes.
In general, primary conversion processes allow for direct conversion of excess electricity into a desirable output product in a single step. This conversion typically occurs via an electrochemical process known as electrolysis, which can be applied to numerous starting materials and final products. For example, water can be electrolyzed to produce hydrogen. CO2 can be electrolyzed into a variety of products; particularly CO. Nitrogen from air can be electrolyzed into ammonia. Oxygen can be electrolyzed into hydrogen peroxide. The list of such potential primary conversion processes is long and varied. However, many of these processes (such as CO2 and nitrogen electrolysis) are relatively immature compared to conventional thermochemical processes that produce the same products. Furthermore, not all possible products are equally attractive from a financial or utilitarian perspective.
Secondary conversion processes use the outputs from primary conversion processes as inputs to yield more valuable final products. Typically, secondary conversion processes are much more mature than alternative primary processes, since they have benefitted from years of development and scaling with fossil fuel-based inputs. As a result, combining a few mature and versatile primary conversion processes with well-established secondary conversion processes is currently more effective for industrial scale-up of PtX products than focusing on a larger number of immature, specialized primary conversion technologies. Because the secondary conversion processes are running on green output from primary PtX processes, they are effectively decoupled from fossil fuels despite being originally developed for fossil fuel-derived inputs.
In this context, water-to-hydrogen electrolysis has emerged as the most attractive primary conversion process to pursue for scale-up. This is because hydrogen can be used as a fuel on its own or fed into multiple well-stablished secondary conversion processes to produce other attractive products (methane, methanol, longer liquid hydrocarbons, ammonia). After water electrolysis, CO2 electrolysis is likely the next most attractive technology for scale-up, as the CO it provides can be used to make a wide variety of other products via similar established secondary conversion processes.
Electrolyzers, fuel cells, and the importance of slot-die coating
For PtX production of these desirable fuels to reach industrial scale, water electrolysis needs to be developed to an industrial level. Furthermore, water electrolyzers need to be optimized and manufactured quickly and to a high standard, including new electrolzyer designs that enable improved performance and durability compared to well-known alkaline electrolyzer technology.

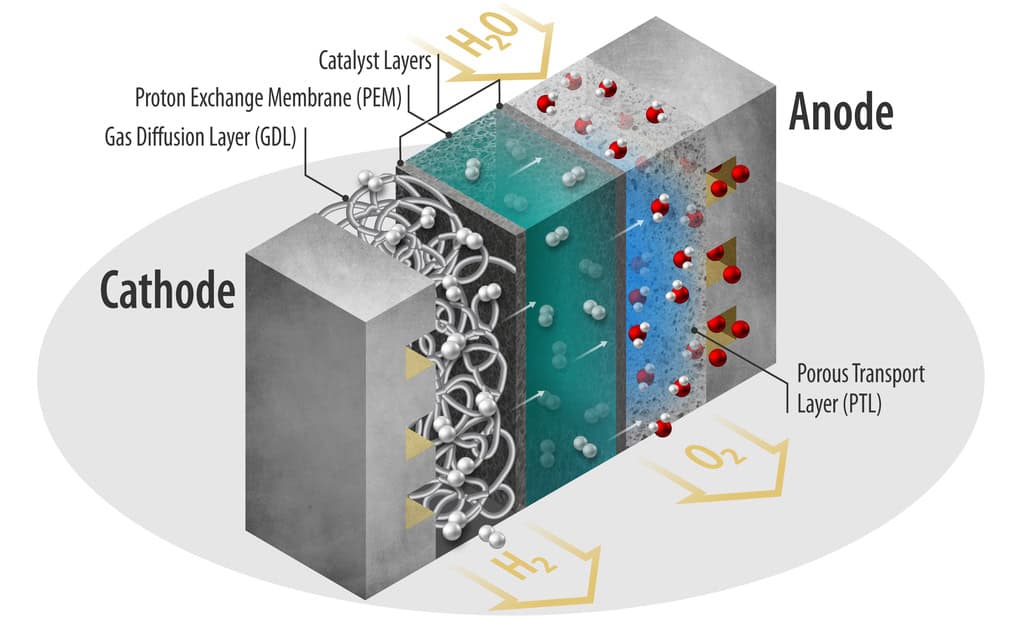
Three prevalent water electrolyzer designs. From left to right: alkaline electrolyzer, proton exchange membrane electrolyzer, and solid oxide electrolyzer.
Looking to more long-term developments; other primary conversion processes (such CO2 and nitrogen electrolysis) are likely to receive increased R&D attention in efforts to expand the flexibility and efficiency of PtX-based chemical production in the future. Furthermore, a diverse range of fuel cell technologies could well experience a similar boost, in order to take advantage of the shift towards abundant new PtX-based fuels like methanol and ammonia, which are not as amenable to combustion in conventional engines as fossil fuels.
While the exact composition of our future energy infrastructure is still being determine, we at FOM Technologies are thrilled to be at the heart of yet another critical technology enabling the Green Transition. If you’re interested in exploring slot-die coating for your own R&D or scale-up efforts related to electrolyzers and fuel cell technologies, don’t hesitate to get in touch!

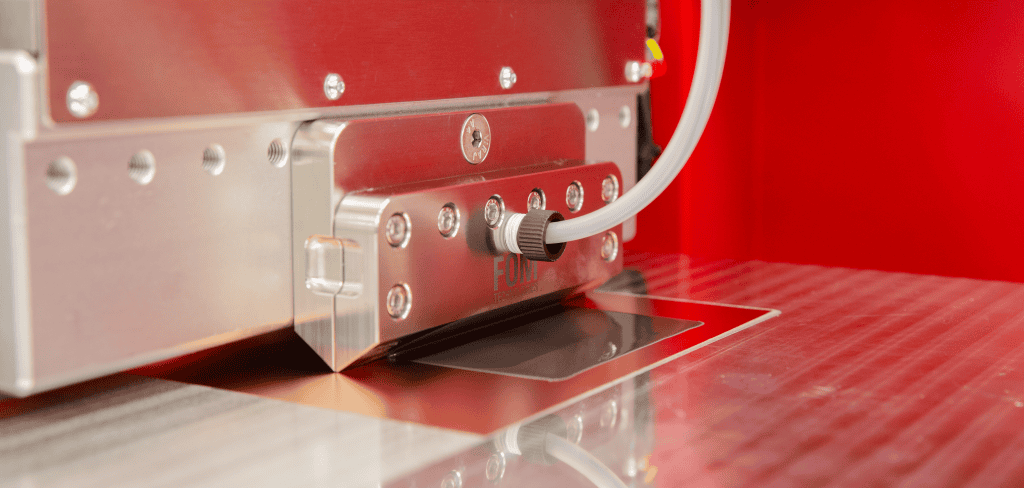
Electrolyzer stack coatings achieved in collaboration with the Madsen research group at the University of Southern Denmark Mads Clausen Institute using a FOM alphaSC slot-die coater.
Read more on the topic
Download the FOM Technologies Product brochure
Fill in your information and click download to access the pdf.